Green hydrogen
The tipping point towards carbon-free energy
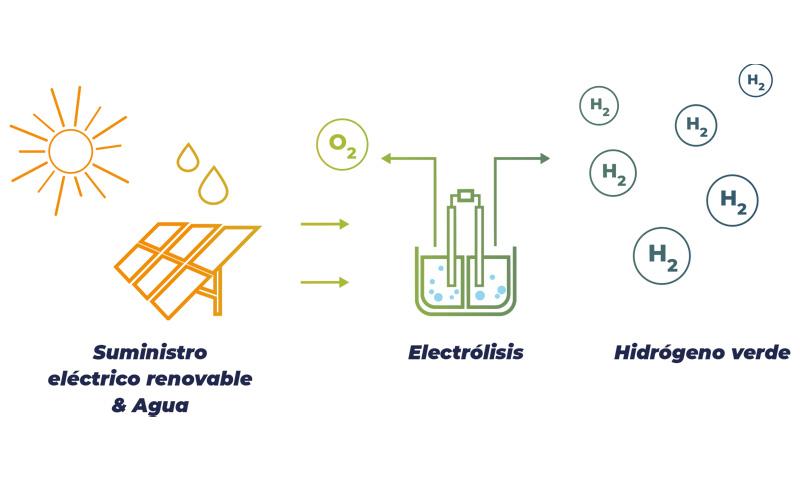
Hydrogen production with renewable energy through water electrolysis
Pure hydrogen is only available in small quantities in the Earth. Hydrogen can be produced with fossil fuels in a process that emits large quantities of CO2 into the atmosphere. Hydrogen can also be produced through water electrolysis, a process that was invented by two British chemists in 1800: 2 H2O + electricity = 2 H2 + O2 + heat. Electrical activation between a cathode and an anode creates a chemical reaction. Energy content of water is considerable: 1 liter of water contains the equivalent to 0.4 liter of oil.
Upstream
Equalizing the cost
of fossil fuels
Green hydrogen can be produced at competitive prices.

Midstream
Use of existing oil and gas infrastructure
Gas transmission and storage infrastructure, as well as coal and gas turbines, can be adapted and reused.

Downstream
Satisfying the energy need of the industry 24 hours per day, 7 days per week. Energy, heat and transport
The chemical industry, the steel manufacturing, energy generation, road and sea transportation, they can all transit to hydrogen.

Energy without carbon emissions, competitive and secure is an imperative
The arctic melting, the burning of the Amazon forest, the rise of the sea level… There is only one answer: stop fossil fuels now! The European Union has set a target of carbon neutrality by 2050.